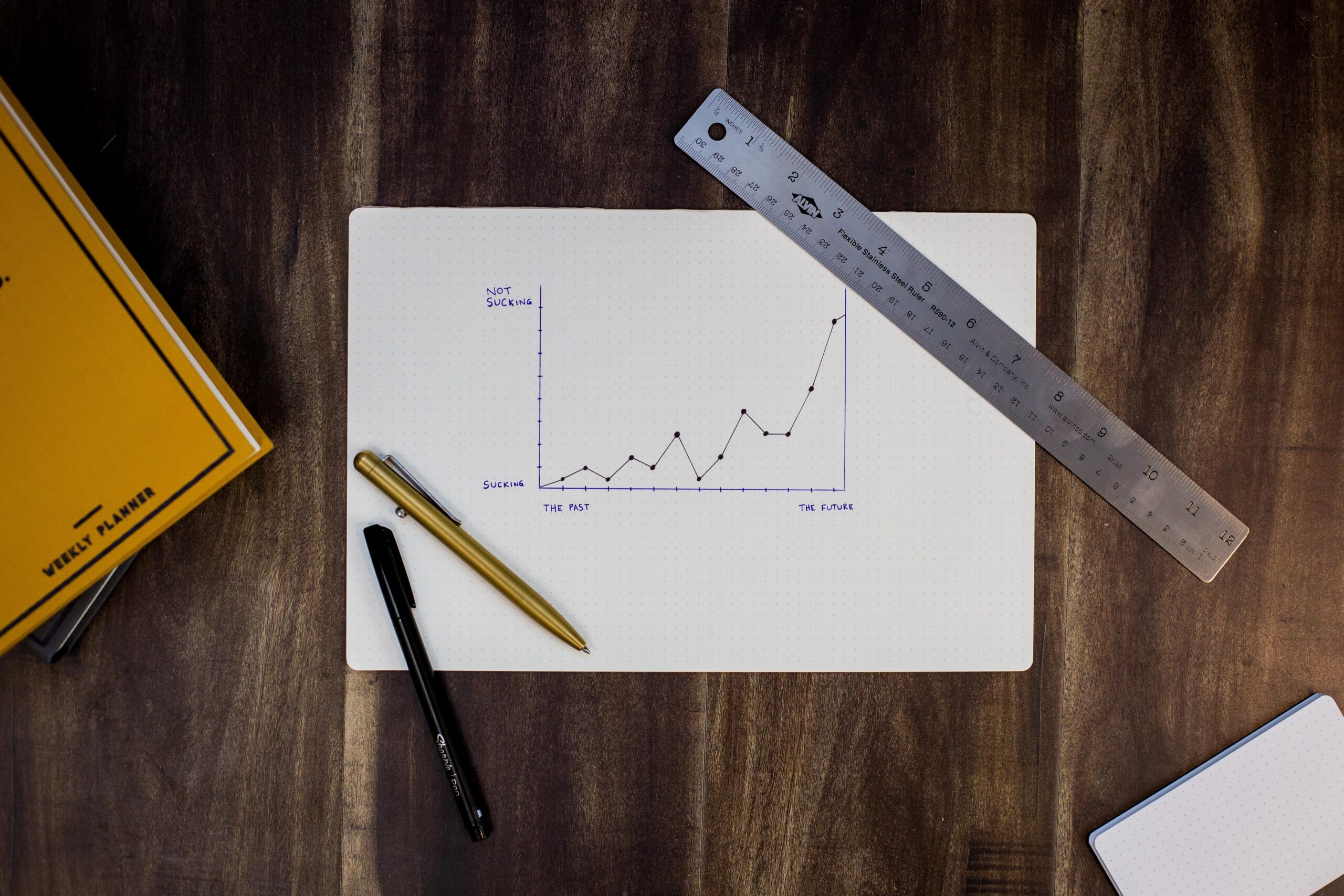
SEMrush Quick Review
February 4, 2021
Enabling logs in wp-config
February 23, 2021How to use the “Naranjo Scale”. This scale is for determining the adverse drug reaction probability; it was designed by Naranjo et al.
The scale consists of series of questions that will give the healthcare provider an idea about the likelihood of whether the ADR was a result of the drug itself, or some other factors behind the scene.
The Naranjo criteria classify the probability that an adverse event is related to drug therapy based on a list of weighted questions, which examine factors such as the temporal association of drug administration and event occurrence, alternative causes for the event, drug levels, dose-response relationships and previous patient experience with the medication. The ADR is assigned to a probability category from the total score as follows:
- definite if the overall score is 9 or greater
- probable for a score of 5-8
- possible for 1-4
- doubtful if the score is 0
The Naranjo criteria doesn’t take into account drug-drug interactions. Drugs are evaluated individually for causality, and points deducted if another factor may have resulted in the adverse event, thereby weakening the causal association.
Use the calculator below to get the Naranjo Scale:






